ASEI Lesson Plan and Note Template on Chemistry
Lesson Note and Plan on Reduction and Oxidation Reaction
Theme: Types of Reaction
Topic: Reduction & Oxidation Reaction
Sub Topic: Redox Reaction
Date: dd/mm/yyyy
Class: S.S.S 2
Duration: 35 Minutes
No of Learners: 30
Learning Objectives:
By the end of the lesson learners should be able to:Identify the ion and oxidation number of an element/ compound by figuring out the protons, neutrons, and electrons of the element in the periodic table of element:
Explain the redox reactions in terms of:
- REDUCTION is Losing (Removal) of Oxygen and OXIDATION is Gaining (Addition) of Oxygen Reduction Reaction is defined as the loss of oxygen by a compound. E.g.
- the reduction of copper (II) oxide to copper by heating with charcoal (carbon).
2CuO(s) + C → 2Cu + CO₂ - the reaction of copper (II) oxide and hydrogen, copper (II) oxide is reduced to copper after oxygen has been removed.
CuO + H₂ → Cu + H₂O - the reaction of magnesium with steam whereby steam is reduced to hydrogen by the removal of oxygen.
Mg + H₂O(g) → MgO + H₂ - burning carbon to produce carbon dioxide is oxidation.
C + O₂ → CO₂ - the reaction of a copper atom with oxygen, the copper atom is oxidised to copper (II) oxide.
2Cu + O₂ → 2CuO - the reaction of hydrogen with oxygen to form the compound water.
2H₂ + O₂ → 2H₂O - REDUCTION is Gaining (Addition) Hydrogen and OXIDATION is Losing (Removal) of Hydrogen A reduction reaction is defined as the addition of hydrogen to a substance. E.g.
- the reaction of hydrogen with oxygen to form the compound water.
2H₂ + O₂ → 2H₂O - the reaction of hydrogen sulphide with chlorine, the addition of hydrogen to chlorine is a reduction reaction.
H₂S(g) + Cl₂(g) → 2HCl(g) + S(s) - the reaction of hydrogen with magnesium oxide, the magnesium (II) oxide is reduced to magnesium by the addition of hydrogen.
MgO + H₂(g) → Mg + H₂O - REDUCTION is the Removal of an Electronegative element as well as the addition of an Electropositive element and OXIDATION is the addition of electronegative elements and the removal of electropositive elements.
- REDUCTION is the gaining of electrons and OXIDATION is the loss of electrons.
Identify oxidizing and reducing agents.
- Oxidizing agents: are substances or chemical species containing the element or species that accepts electrons allowing another element or species to be oxidized
By accepting electrons, the element or species in the oxidizing agent are reduced. - Reducing agents: are substances or chemical species containing the element or species that donate electrons, allowing another element or species to be reduced.
By giving up electrons, the element or species in the reducing agent are oxidised. State the rules used to determine oxidation numbers of elements in chemical compounds and species and calculate/ assign the oxidation number of an element in a compound.
- The oxidation number of an element in its elemental form is 0 (zero). For example,
Na°, Cl₂°, Mg°, P₄°. The zero, however, is often omitted. - In compounds, the oxidation number of oxygen is almost always –2. The most common exception is in peroxides when the oxidation number is –1. Peroxides are compounds having two oxygen atoms bonded together. For example, hydrogen peroxide is H-O-O-H. In hydrogen peroxide, each oxygen atom has a ‐1 oxidation number. When Oxygen is bonded to fluorine, as in hypofluorous acid (HOF), the oxidation number of oxygen is 0. Oxygen‐fluorine compounds such as OF₂, the oxidation number of oxygen is +2
In superoxide, O2-, we see that each oxygen atom bears a -1/2 oxidation state. - The oxidation number of a simple ion (i.e. an ion consisting of a single element) is equal to the charge on the ion.
The oxidation number of Na⁺, Mg²⁺, Al³⁺, Cl⁻ and S²⁻ are +1, +2, +3, -1 and -2 respectively.
Note that the negative or positive sign for an oxidation number always comes before the number, the “+” and “- “signs are just as important as the number (they indicate the nature of the charge). - The oxidation numbers of group 1 and group 2 elements are +1 and +2 respectively.
- In compounds, the oxidation number of hydrogen is almost always +1. The most common exception occurs when hydrogen combines with metals; in this case, the oxidation number of hydrogen is typical –1.
- In compounds, the oxidation number of fluorine is always –1. The oxidation number of other halogens (Cl, Br, I) is also –1, except when they are combined with oxygen. The oxidation state of halogens (except fluorine) combined with oxygen is typically positive. For example, in ClO⁻, chlorine’s oxidation number is +1.
- For a complex ion, the sum of the positive and negative oxidation numbers of all elements in the ion equals the charge on the ion; e.g. SO₄²⁻ i.e [-2 = +6 + 4(-2)], OH⁻ i.e [-1 = -2 + (+1)], the oxidation numbers of NH₄⁺, NO₃⁻, PO₄³⁻ and MnO₄⁻ are +1, -1, -2, -3 and -1 respectively.
- For an electrically neutral compound, the sum of the positive and negative oxidation numbers of all elements in the compound equals zero. In any compound, the more electronegative atom has the negative oxidation number while the less electronegative atom has the positive oxidation number. For example in SCl₂, sulfur has an oxidation number of +2 since it is less electronegative than chlorine but in sodium sulphide, Na₂S, its oxidation number is -2.
- The sum of the oxidation numbers is 0 for a molecule and is equal to the net charge for a polyatomic ion.
In the compound MgCl₂, which may also be written as (MgCl₂)° [0 = +2 + 2(-1)]
This rule is particularly useful for finding the oxidation number of an atom in any chemical compound or species.
The general procedure is to assign oxidation numbers to the “easy” or known atoms first and then find the unknown oxidation number by calculation.
For example,- Find the oxidation number of the sulfur atom in sulfuric acid (H₂SO₄). Solution
- Find the oxidation number of the manganese atom in potassium tetraoxomanganate(VII), KMnO₄ Solution
- Find the oxidation number of the chromium atom in potassium heptaoxodichromate(VI), K₂Cr₂O₇. Solution
- Find the oxidation number of the chlorine atom in the perchlorate anion (ClO₄⁻). Solution
Since each H atom is +1 and each O atom bears -2, the S atom must have an oxidation number of +6 for the compound to have no net charge: 2(+1) + (?) + 4(–2) = 0 net charge
? = 0 – 2(+1) – 4(–2) = + 6
To find the oxidation number of the chlorine atom in the perchlorate anion (ClO₄⁻), we know that each oxygen has a charge of -2, so the Cl atom must have an oxidation number of +7 for there to be a net charge of –1 on the ion:
? + 4(–2 ) = –1 net charge
? = –1– 4(–2) = +7
(Oxidation number of K) + (Oxidation number of Mn) + 4(Oxidation number of O) = 0
oxidation number of K = +1,
oxidation number of Mn = X,
oxidation number of O = -2,
:. (+1) + X + [4 x (-2)] = 0
1 + X - 8 = 0
X = +7
The oxidation number of the manganese atom in potassium tetraoxomanganate(VII) is +7.
2(Oxidation number of K) + 2(Oxidation number of Cr) + 7(Oxidation number of O) = 0
oxidation number of K = +1,
oxidation number of Cr = X,
oxidation number of O = -2,
:. 2(+1) + 2(X) [7 x (-2)] = 0
2 + 2X - 14 = 0
2X = +12
X = +6
The oxidation number of the chromium atom in potassium heptaoxodichromate(VI) is +6.
each oxygen has a charge of -2, so Cl atom must have an oxidation number of +7 for there to be a net charge of –1 on the ion:
? + 4(–2 ) = –1 net charge
? = –1– 4(–2) = +7 Balancing redox reactions (equations) in aqueous/neutral solutions, acidic solutions and basic solutions using the Half Reaction Method.
In redox reaction using the half-reaction method, it is useful to separate the reaction into two half-reactions one involving oxidation reaction and the other involving reduction reaction. Then after balancing those half-reactions, find the overall oxidation-reduction (redox) reaction by summing up the two half-reactions
NOTE: In balancing redox reaction using the half-reaction method, you need to make sure that not only the particles, atoms and ions are balanced but also the chargers must be balanced on both sides.
- The Half-Reaction Method for Balancing Oxidation-Reduction Reactions in Aqueous Solutions. For example, Balance the following equation of the reaction which occurs in aqueous solutions.
- The number of Nickel particles is the same on both sides.
- The number of the Aluminium particle are the same on both side.
- The net charge on the left is 3 x +2 = +6, and the net charge on the right is 2 x +3 = +6, hence they are the same on both sides. The number of atoms is the same on both sides and the number of charges is also the same on both side.
- The Half-Reaction Method for Balancing Oxidation-Reduction Reactions in Acidic Solutions.
- Write separate equations for the oxidation and reduction half-reactions.
- For each half-reaction,
- balance all the elements except hydrogen and oxygen.
- then balance oxygen using H₂O.
- then balance hydrogen using H⁺.
- then balance the charge using electrons.
- If necessary, multiply one or both balanced half-reactions by an integer to equalize the number of electrons transferred in the two half-reactions.
- Add the half-reactions, and cancel identical species.
- Checking that the elements and charges are balanced.
- one bromine atom on both side
- Three zinc particles on both side
- Six hydrogen atoms on both side
- The net charge on the left is -1 + 6(+1) = +5, and the net charge on the right is 3(+2) + (-1) = +5, hence they are the same on both sides. The number of particles is the same on both side and the number of charges is also the same on both side.
- The half-reaction method for balancing equations for oxidation-reduction reactions occurring in basic solution.
- Write separate equations for the oxidation and reduction half-reactions.
- For each half-reaction,
- balance all the elements except hydrogen and oxygen.
- then balance oxygen using H₂O.
- then balance hydrogen using H⁺.
- addition of some OH- ions equal to the number of H+ ions to each side of each half-equation.
- then balance the charge using electrons.
- If necessary, multiply one or both balanced half-reactions by an integer to equalize the number of electrons transferred in the two half-reactions.
- Add the half-reactions, and cancel identical species.
- Checking that the elements and charges are balanced.
- 8 aluminium atoms on both sides
- 3 chlorine atoms on both sides
- 32 hydrogen atoms on both sides
- 32 oxygen atoms on both sides
- The net charge on the left is 8(-1) + 3(-1) = -11, and the net charge on the right is 3(-1) + 8(-1) = -11, hence they are the same on both sides. The number of particles is the same on both side and the number of charges is also the same on both side.
Al + Ni²⁺ → Al³⁺ + Ni
Solution
There is an equal (one) Aluminium atom on both sides.
There is an equal Nickel particle on both sides, hence, the atoms are balanced.
There is a net charge of +2 ion on the left and a net charge of +3 ion on the left, hence the charges are not balanced.
Separate the equation into half-reaction.
Al → Al³⁺ (net charge on the left = 0, net charge on the right = +3)
Difference between charges +3 - (0) = 3, add 3 electrons to the side with the highest charges
Al → Al³⁺ + 3e‾ This half-reaction is now balanced (Whenever we have the electron (e‾) on the right of the reaction, it represents the oxidation half-reaction)
Ni²⁺ → Ni (net charge on the left = +2, net charge on the right = 0)
Difference between charges +2 - (0) = 2, add 2 electron to the side with the highest charges
Ni²⁺ + 2e‾ → Ni This half-reaction is now balanced (Whenever we have the electron (e‾) on the left of the reaction, it represents a reduction in half-reaction)
Before adding both half-reactions, make sure the numbers of electrons are the same on both sides, the least common multiple of 2 and 3 is 6, hence, we need a total of 6 electrons on both sides of the half-reactions.
2(Al → Al³⁺ + 3e‾)
3(Ni²⁺ + 2e‾ → Ni)
2Al → 2Al³⁺ + 6e‾
3Ni²⁺ + 6e‾ → 3Ni
Now add both half reactions (Note, the 6e‾ on both side cancel out)
2Al + 3Ni²⁺ → 2Al³⁺ + 3Ni
Check that the elements and charges are balanced.
- STEPS
Zn + BrO₃‾ → Zn²⁺ + Br¹‾
Solution
There is an equal (one) Zinc atom on both sides.
There is an equal Bromine particle on both side.
There is an unequal Oxygen element on both sides.
Hence, the atoms are not balanced.
There is a net charge of -1 ion on the left and a net charge of +1 (i.e +2 - (-1) = +1) ion on the left, hence the charges are not balance
Separate the equation into half-reaction.
Zn → Zn²⁺ (net charge on the left = 0, net charge on the right = +2)
Difference between charges +2 - (0) = 2, add 2 electron to the side with the highest charges
Zn → Zn²⁺ + 2e‾ This half-reaction is now balanced (oxidation half-reaction).
BrO₃‾ → Br¹‾ Net charge on the left = -1, net charge on the right = -1, they are balanced.
We have equal bromine atoms on both sides but unequal Oxygen atoms (3 molecules of Oxygen on the left null on the right), to balance the oxygen atom add 3 water molecule to the right side of the reaction.
BrO₃‾ → Br¹‾ + 3H₂O There are 3 Oxygen atoms on the left and 3 Oxygen atoms on the right, there are null Hydrogen atoms on the right but 6 Hydrogen atoms on the left, add 6 Hydrogen atoms to the left.
BrO₃‾ 6H⁺ → Br¹‾ + 3H₂O All atoms on both sides are now balanced. Net charge on the left is -1 + 6(+1) = +5, net charge on the right is -1. Difference between the net charges +5 - (-1) = 6 :. add 6 electrons to the side with the highest charge.
BrO₃‾ 6H⁺ + 6e‾ → Br¹‾ + 3H₂O Now make the number of electrons equal on both sides, the least common factor of 2 that will give us 6 is 3, hence, we need a total of 6 electrons on both sides of the half-reactions.
3(Zn → Zn²⁺ + 2e‾)
3Zn → 3Zn²⁺ + 6e‾
BrO₃‾ 6H⁺ + 6e‾ → Br¹‾ + 3H₂O Now add both half reactions (Note, the 6e‾ on both side cancel out)
3Zn + BrO₃‾ + 6H⁺ → 3Zn²⁺ + Br¹‾ + 3H₂O
Check that the elements and charges are balanced.
- STEPS
Al + ClO₄‾ → Al(OH)₄‾ + Cl‾
Solution
There is an equal Aluminium atom on both sides
There is an equal Chlorine atom on both sides
There is an equal Oxygen atom on both sides
There is an unequal Hydrogen element on both sides
Hence, the atoms are not balanced
There is a net charge of -1 ion on the left and a net charge of 0 [i.e -1 + (-1) = 0] ion on the right, hence the charges are not balance
Separate the equation into half-reaction
Al → Al(OH)₄‾There is an unequal number of hydroxide molecules (4 molecules of Oxygen and 4 molecules of Hydrogen on the right, there are null of Hydrogen and Oxygen on the left, add 4 Hydroxide molecules to the right).
Al + 4OH‾ → Al(OH)₄‾ (net charge on the left = -4 i.e 4 x -1 = -4, net charge on the right = -1) Difference between charges -4 - (-1) = -3, add 3 electron to the side with the highest charges
Al + 4OH‾ → Al(OH)₄‾ + 3e‾ This half-reaction is now balanced (oxidation half-reaction).
ClO₄‾ → Cl‾ There are an unequal number of Oxygen atoms on the right (4 molecules of Oxygen on the left, null on the left). First, we right this reaction under acidic conditions by adding four molecules of water.
ClO₄‾ → Cl‾ + 4H₂O There is 4 Oxygen atom on the left and 4 Oxygen atom on the right, there are null Hydrogen atom on the right but 8 Hydrogen atom on the right, add 8 moles of Hydrogen ion to the left to balance the hydrogen atoms on both sides.
ClO₄‾ + 8H⁺ → Cl‾ + 4H₂O On the basic condition the H⁺ does not exist so we have to get rid of it by adding 8OH‾ to both sides.
ClO₄‾ + 8H⁺ + 8OH‾ → Cl‾ + 4H₂O + 8OH‾ The 8H⁺ + 8OH‾ molecule will combine to form 8 water molecules.
ClO₄‾ + 8H₂O → Cl‾ + 4H₂O + 8OH‾ The 8H₂O on the right and the 4H₂O on the left can be simplified by subtracting 4H₂O molecule from both sides.
ClO₄‾ + 8H₂O - 4H₂O → Cl‾ + 4H₂O - 4H₂O + 8OH‾
ClO₄‾ + 4H₂O → Cl‾ + 8OH‾ The number of atoms on both side are now balanced, now check the net charges on both sides. The the left the net charges is -1, on the right the net charges is -9 [i.e -1 + (-8) = -9], difference between the net charges on the right and left -1 - (-9) = 8, add 8 electron to the side with the highest charges
ClO₄‾ + 4H₂O + 8e‾ → Cl‾ + 8OH‾ Reduction half-reaction
Al + 4OH‾ → Al(OH)₄‾ + 3e‾ Oxidation half-reaction
Make the number of electrons on the reduction half-reaction and the number of electrons on the oxidation half-reaction equal. The least common multiple of 8e‾ and 3e‾ is 24, hence, add 8 to the oxidation reaction and 3 to the reduction reaction.
3(ClO₄‾ + 4H₂O + 8e‾ → Cl‾ + 8OH‾)
8(Al + 4OH‾ → Al(OH)₄‾ + 3e‾)
8Al + 32OH‾ → 8Al(OH)₄‾ + 24e‾
3ClO₄‾ + 12H₂O + 24e‾ → 3Cl‾ + 24OH‾ Now add both half reactions (Note, the 24e‾ on both side cancel out)
8Al + 32OH‾ + 3ClO₄‾ + 12H₂O → 8Al(OH)₄‾ + 3Cl‾ + 24OH‾
Simplify 32OH‾ on the left and 24OH‾ on the right by subtracting 24OH‾ from both side
8Al + 32OH‾ + 24OH‾ + 3ClO₄‾ + 12H₂O → 8Al(OH)₄‾ + 3Cl‾ + 24OH‾ + 24OH‾
8Al + 8OH‾ + 3ClO₄‾ + 12H₂O → 3Cl‾ + 8Al(OH)₄‾
Check that the elements and charges are balanced.
Therefore, 8Al + 8OH‾ + 3ClO₄‾ + 12H₂O → 3Cl‾ + 8Al(OH)₄‾
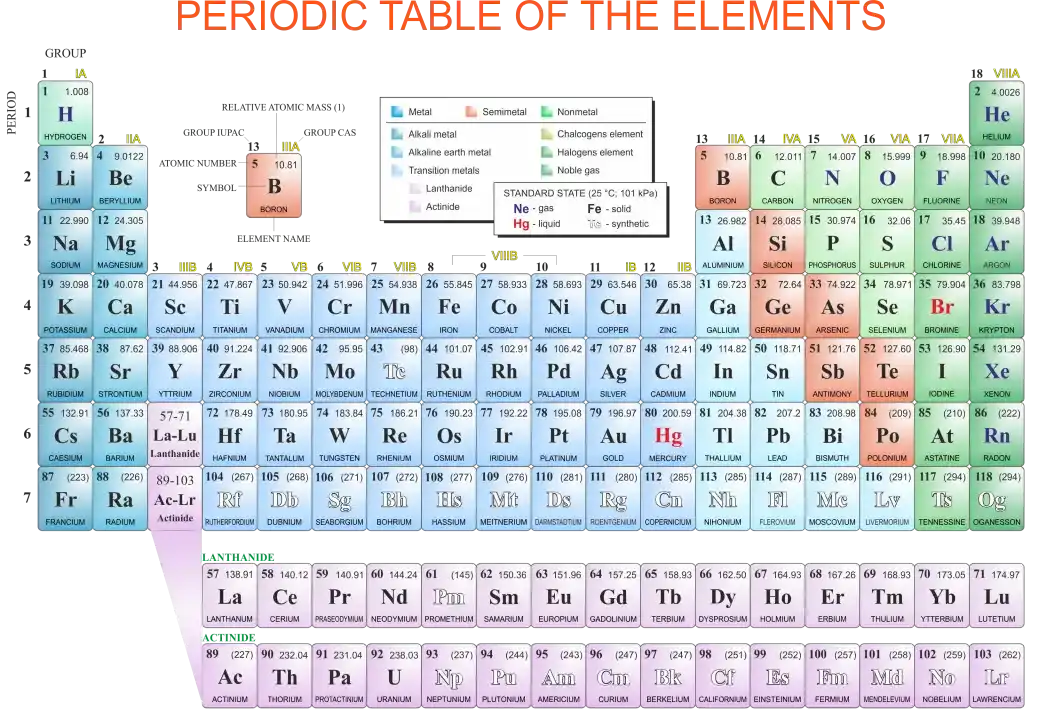 Modern Periodic Table
Modern Periodic Table
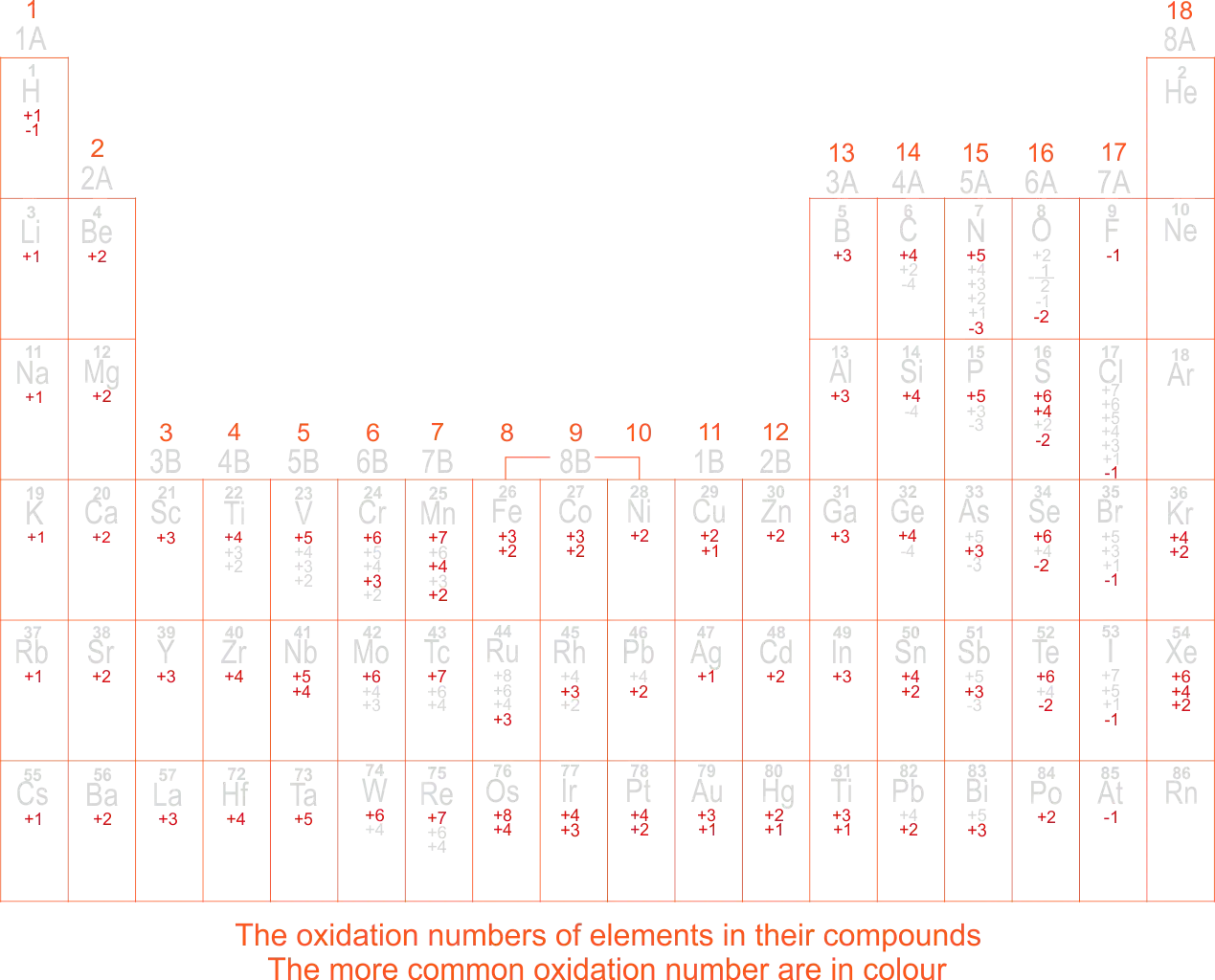 The Oxidation Numbers of Elements in their Compounds in Modern Periodic Table
The Oxidation Numbers of Elements in their Compounds in Modern Periodic Table
| Oxidation | Reduction | ||
|---|---|---|---|
| Gaining oxygen | Losing oxygen | ||
| Losing hydrogen | Gaining hydrogen | ||
| Addition of electronegative elements & removal of electropositive elements | Removal of electronegative elements & addition of electropositive elements | ||
| Losing electron | Gaining electron | ||
Oxidation Reaction is defined as the reaction where substances are combined with the element oxygen. E.g.
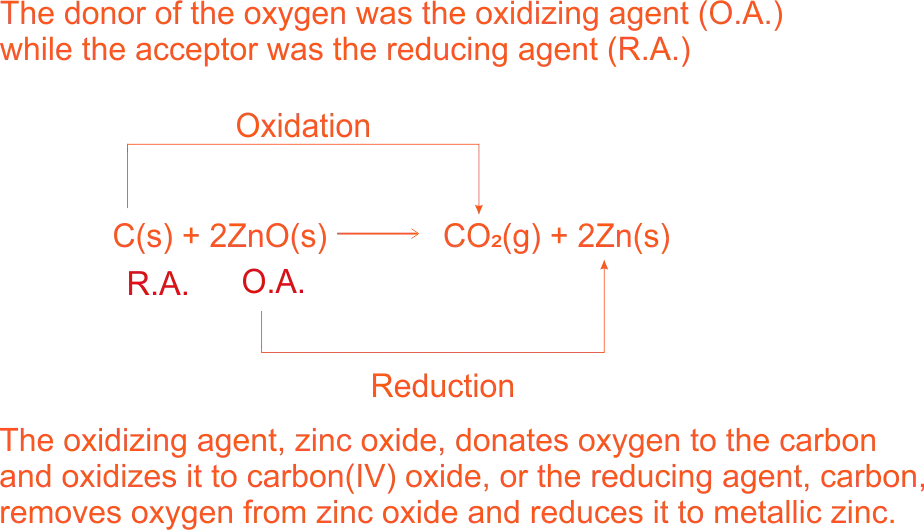 Oxidation of Oxygen sulphide
Oxidation of Oxygen sulphide
Oxidation Reaction is defined as the removal of hydrogen from a substance. E.g.
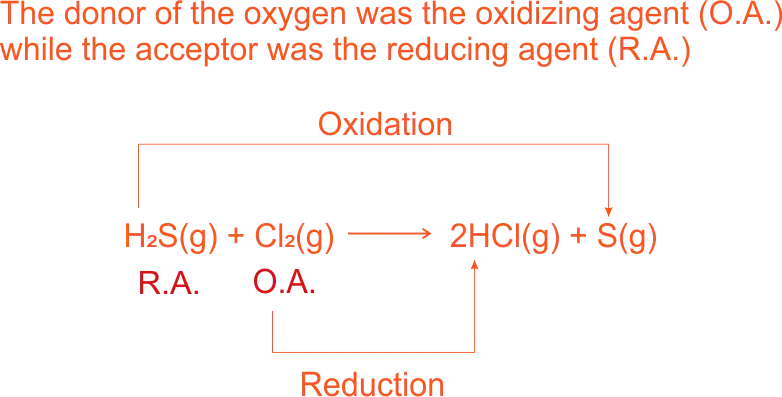 Oxidation of hydrogen sulphide
Oxidation of hydrogen sulphide
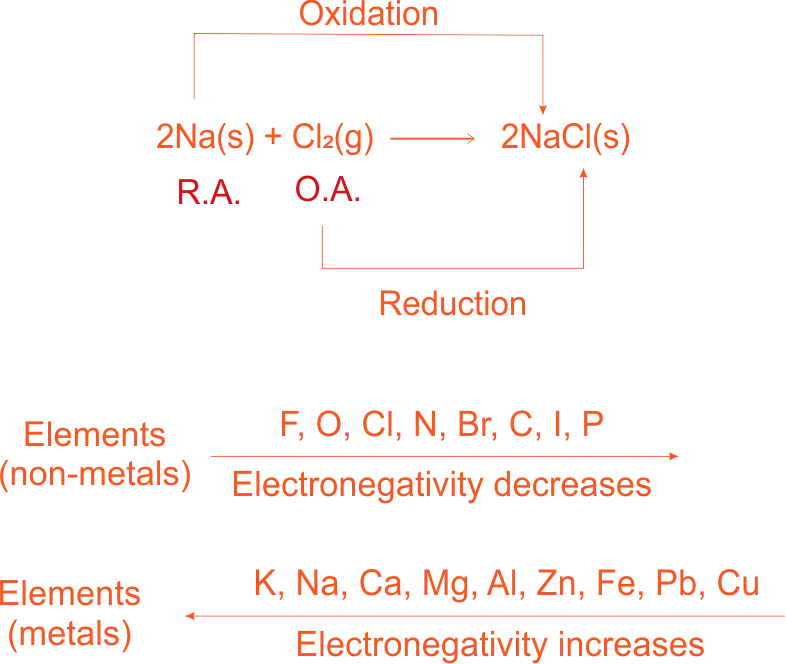 Electronegetivity of Metals and Non-Metals
Electronegetivity of Metals and Non-Metals
Electronegative elements tend to gain electrons, e.g. non-metals like chlorine.
Electropositive elements tend to lose electrons and become positive ions, e.g. metals of sodium.
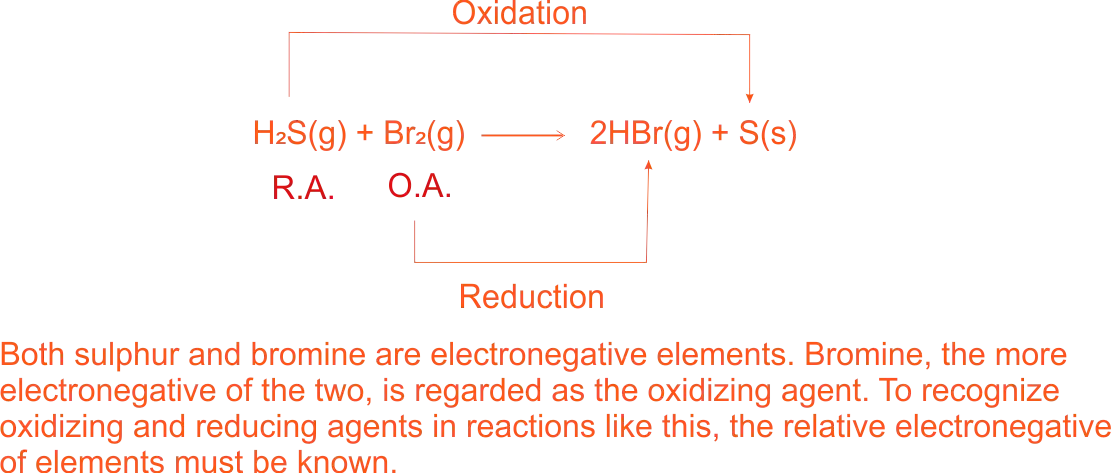 Electronegetivity of Bromine and Sulphur
Electronegetivity of Bromine and Sulphur
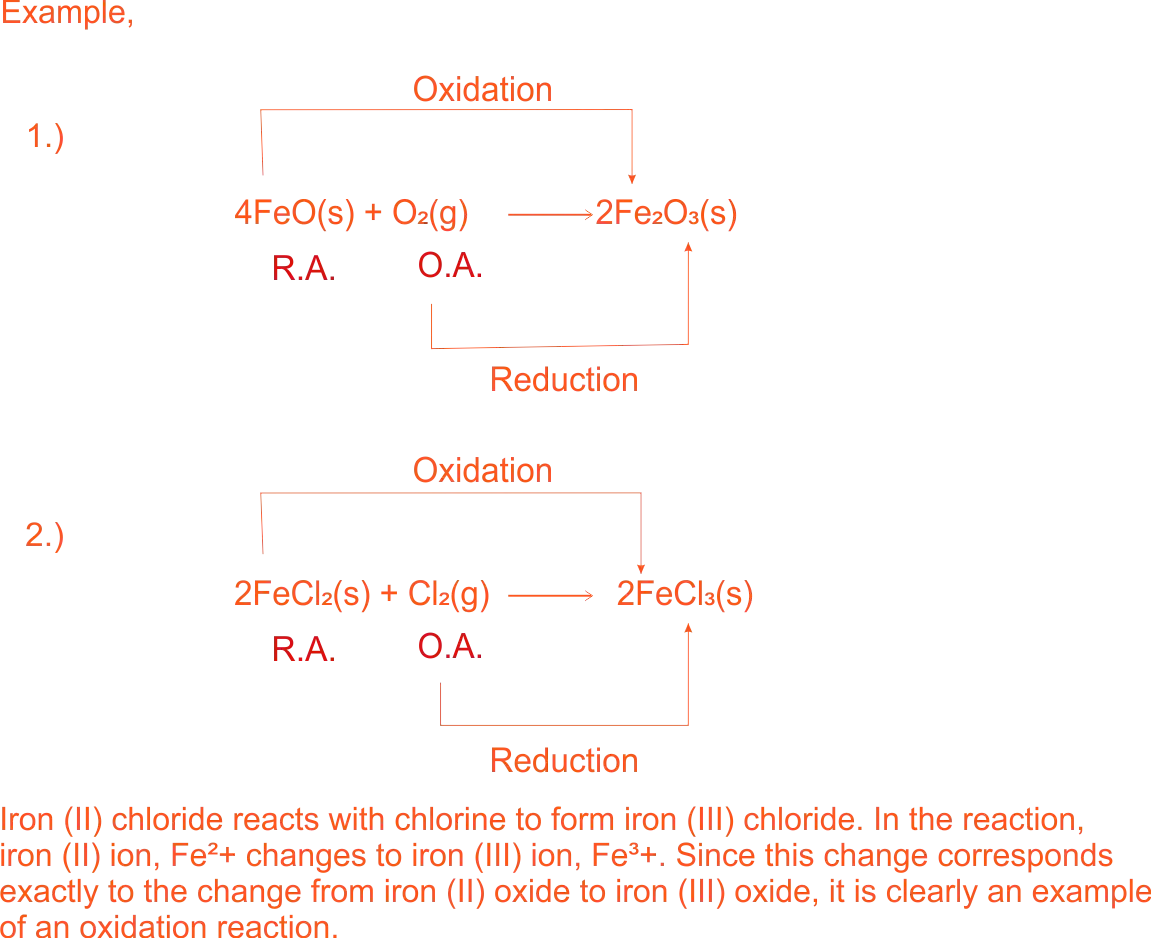 Increase in oxidation of Metal
Increase in oxidation of Metal
2KI(aq) + Fe₂(SO₄)₃(aq) → I₂(s) + K₂SO₄(aq) + 2Fe(aq)
Ionically,
2I⁻(aq) + 2Fe³⁺(aq) → I₂(s) + 2Fe²⁺(aq)
In this reaction, iodide ions are oxidized to iodine molecules and iron(III) ions are reduced to iron(II) ions
Similarly, reactions where copper(I) changes to copper(II), lead(II) to lead(IV) etc, are all regarded as oxidation reactions. In all these changes, the charge on a positive ion is increased, e.g.
Cu⁺ → Cu²⁺,
while the charge on a negative ion is decreased, e.g.
I⁻¹ → I°.
A substance loses electrons when it is oxidized and gains electrons when it is reduced, while a redox reaction is a transfer of electrons from the reducing agent (the electron donor) to the oxidizing agent (the electron acceptor).
| Reducing agent | Oxidizing agent | ||
|---|---|---|---|
| Causes reduction by donating electron(s) | Causes oxidation by removing electron(s) from others | ||
| Loses one or more electrons | Gains one or more electrons | ||
| Undergoes oxidation; i.e. oxidation number of atoms increases | Undergoes reduction, i.e.oxidation number of atoms decreases | ||
In general, metals give up electrons and act as reducing agents, while reactive nonmetals such as O₂ and halogens accept electrons and act as oxidizing agents.
Rationale:
When we talk about chemical reactions, we usually discuss the breakage and formation of bonds, gain and loss of electrons, and conversion from one state of matter to another. If we look closely, we might observe hundreds of chemical reactions taking place in our vicinity. You may find it quite surprising that almost one-third of the chemical reactions taking place in the surroundings fall under the category of redox reactions. E.g. The rusting of iron. The food that we consume is converted into energy by redox reactions. In photosynthesis, water is oxidised and carbon dioxide is reduced. The process of developing a photographic film also employs redox reactions, a positive image is obtained by the exposure of the negative to light. Following exposure to light, silver cations are reduced. The living matter in nature, primarily, is made up of carbon, hydrogen, and oxygen. Upon the death of any living organism, the organic compounds present in the organism start reacting with oxygen. This process commonly referred to as “decay” or “decomposition” forms another example of redox reactions.
Prerequisite/ Previous knowledge:
Types of chemical reactions.Learning Materials:
Iron powder, 2M sulphuric acid, Bunsen burner, hydrogen peroxide (20 cm3), filter paper, test-tube and test-tube racks, 2M sodium hydroxide, filter funnel, 2M ammonia solution. The periodic table of metals.
Reference Materials:
New School Chemistry. By Ababio
Lamlad's S.S.C.E and UTME by F. O. Ayinde and F. O. I. Asubiojo
Lesson Development:
| STAGE | TEACHER'S ACTIVITY | LEARNER'S ACTIVITY | LEARNING POINTS | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INTRODUCTION full class session (5mins) |
The teacher begins the lesson by reviewing the types of chemical reactions. Chemical reactions are changes in which some new chemical substances are formed. The substances which undergo the chemical change are known as reactants, while the new substances formed are called products. The following are types of chemical reactions;
Promoters and inhibitors:Promoters improve the efficiency of a catalyst while inhibitors or catalyst poisons inhibit a catalyst. E.g, Hydrogen sulphide, arsenic(III) oxide, Hydrogen cyanide and Mercury salts are catalyst poisons which harm us by inhibiting important enzymes in our bodies. |
The learners listen to the teacher's review and ask questions and make a contribution where necessary. | Reviewing the previous lesson on chemical reactions. | ||||||||||||||||||||||||||||||||||
| STEP 1 5 mins. Development |
The teacher guides learners to explain how a chemical reaction occurs in the following areas:
|
Learners follow teacher guidelines and list and explain the various application of chemical reactions in the environment both in living and non-living things.
1. Application in Cellular Respiration:Cellular Respiration which is the ultimate source of energy in human beings encompasses a series of reactions. The food that we consume is converted into energy. During the process of respiration, the carbon dioxide is reduced whereas the water is oxidised to form oxygen.6CO₂ + 12H₂O + Light energy → C₆H₁₂O₆ + 6H₂O + 6O₂ 2. Application in CombustionIn the burning of organic material and combustion of hydrocarbons in fossil fuels, the oxygen present in the atmosphere bonds with carbon and hydrogen present in the compound being burned. During the process of combustion, the oxygen present in the atmosphere is reduced whereas the compound which is being burned is undergoing oxidation.CₓHᵧ + O₂ → Heat and light + H₂O + CO₂ 3. Application in PhotosynthesisThe process of photosynthesis takes place in the leaves of plants, the carbon dioxide and water combine in the presence of sunlight to release oxygen and glucose. The glucose which is formed in the whole process of photosynthesis is used to fuel the metabolic reactions of the plants. In photosynthesis, water is oxidised and carbon dioxide is reduced.CO₂ + 6H₂O + Energy from light + Chlorophyl in leaves → Heat and light + C₆H₁₂O₆ + 6O₂ 4. Application in CorrosionOxygen atoms present in water oxidise iron (or metal) and, thereby, lead to the generation of free hydrogen ions. The hydrogen ions are generated to combine with oxygen to yield water, and the whole cycle begins once again.Fe + 2H⁺ → Fe²⁺ + H₂ O₂ + 2H₂O → 4OH Fe²⁺ + 2OH‾ → Fe(OH)₂ Fe + CO₂ + H₂O → FeCO₃ + H₂ Fe + H₂S → FeS + H₂ 5. Application in DecompositionThe living matter in nature, primarily, is made up of carbon, hydrogen, and oxygen. Upon the death of any living organism, the organic compounds present in the organism start reacting with oxygen. The aforesaid reaction is a prolonged process. This process is commonly referred to as "decay" or "decomposition".CH₂O (Organic matter) + O₂ → H₂O + CO₂ + Nutrients (NO₃, PO₄, Fe, Si, etc) 6. Application in Bleaching agentA bleaching agent is a substance which can whiten or decolourize other substances. Decolourization of a substance occurs because the electrons move between different energy levels. Any sort of decolourization can be removed by the oxidation of the electrons.Ca(ClO)₂ + 2H₂O → Ca⁺ + 2OH‾ + 2HClO 2HClO + OH‾ → ClO‾ + H₂O HClO + ClO‾ → ClO + Cl‾ + OH ClO + ClO‾ + OH‾ → 2Cl‾ + 2O + OH ClO‾ + OH → ClO + OH‾ |
Developing the concept of the topic, oxidation and reduction. | ||||||||||||||||||||||||||||||||||
The teacher ask learners to identify and group the compounds/ element on learners activity in a tabular form in
The teacher explains to the learners that chemical reactions in which the following occurs are regarded as OXIDATION REACTION
While chemical reactions in which the following occurs are regarded as REDUCTION REACTION
The teacher gives learners A useful mnemonic for redox:- OILRIG: Oxidation Is Loss (of electrons) and Reduction Is Gain (of electrons). Oxidizing agents are always reduced and reducing agents are always oxidized. |
(Hands and Minds-on activity)
|
||||||||||||||||||||||||||||||||||||
| STEP 2 5 mins. Full class discussion |
The teacher asks the learners to explain oxidizing and reducing agents. |
The learners' response to the teacher's question. An element/compound that act as a reducing agent is one that:
|
In general, metals give up electrons and act as reducing agents, while reactive nonmetals such as O₂ and halogens accept electrons and act as oxidizing agents. | ||||||||||||||||||||||||||||||||||
| STEP 3 (5 mins) Group discussion |
The teacher asks learners in group discussions to write a chemical equation for the formation of hydrogen chloride (HCl) and sulfur dioxide (SO₂).
The teacher explains to learners that there is a partial transfer of electrons (from H to Cl in HCl and from S to O in SO 2 ). and asks learners to write the transferred electron of the elements at the superscript on the elements. NOTE: Oxidation numbers enable us to identify elements that are oxidized and reduced at a glance. The elements that show an increase in oxidation number—hydrogen and sulfur in the preceding examples—are oxidized. Chlorine and oxygen are reduced, so their oxidation numbers show a decrease from their initial values. Note that the sum of the oxidation numbers of H and Cl in HCl (+1 and -1) is zero. Likewise, if we add the charges on S (+4) and two atoms of O [2 x (-2)], the total is zero. The reason is that the HCl and SO₂ molecules are neutral, so the charges must cancel. The teacher explains the rules to assign oxidation numbers, and thereafter asks learners to assign oxidation numbers to all the elements in the following compounds and ion: (a) Li₂O, (b) HNO₃, (c) Cr₂O₃²⁻.
|
Learners are expected to respond. H₂(g) + Cl₂(g) → 2HCl(g) S(s) + O₂(g) → SO₂(g) H₂⁰(g) + Cl₂⁰(g) → 2H⁺¹Cl⁻¹(g) S⁰(s) + O₂⁰(g) → S⁺⁴O₂⁻²(g) Remember, all alkali metals have an oxidation number of +1, and in most cases, hydrogen has an oxidation number of 11 and oxygen has an oxidation number of -2 in their compounds. SOLUTION
CHECK In each case, does the sum of the oxidation numbers of all the atoms equal the net charge on the species? |
An atom’s oxidation number, also called oxidation state, signifies the number of charges the atom would have in a molecule (or an ionic compound) if electrons were transferred completely. The numbers above the element symbols are oxidation numbers. In both of the reactions shown, there is no charge on the atoms in the reactant molecules. Thus, their oxidation number is zero. For the product molecules, however, it is assumed that complete electron transfer has taken place and that atoms have gained or lost electrons. The oxidation numbers reflect the number of electrons “transferred.” |
||||||||||||||||||||||||||||||||||
| STEP 4 Balancing Redox Reaction 5 mins |
The teacher asks learners to write a chemical reaction for the formation of magnesium oxide (MgO) from magnesium and oxygen, identifying the gain and loss of ions. The teacher may assist the learners in writing the half-reaction. Magnesium oxide (MgO) is an ionic compound made up of Mg²⁺ and O²⁻ ions. In this reaction, two Mg atoms give up or transfer four electrons to two O atoms (in O₂). For convenience, we can think of this process as two separate steps, one involving the loss of four electrons by the two Mg atoms and the other being the gain of four electrons by an O₂ molecule: |
Learners are expected to respond. 2Mg(s) + O₂(g) → 2MgO(s) 2Mg → 2Mg²⁺ + 4e⁻ O₂ + 4e⁻ → 2O²⁻ 2Mg + O₂ + 4e⁻ → 2Mg²⁺ + 2O²⁻ + 4e⁻ (cancel the electrons that appear on both sides of the equation) 2Mg + O₂ → 2Mg²⁺ + 2O²⁻ (the 2Mg²⁺ and 2O²⁻ ions combine to form MgO) 2Mg²⁺ + 2O²⁻ → 2MgO |
Splitting chemical reactions into two parts, each of these steps is called a half-reaction, which explicitly shows the electrons involved in a redox reaction. The sum of the half-reactions gives the overall reaction. Note: that in an oxidation half-reaction, electrons appear as the product; in a reduction half-reaction, electrons appear as the reactant. balancing redox reactions
|
||||||||||||||||||||||||||||||||||
The teacher explain to learners that Redox reaction could be
The teacher explains the rules to learners. Rules for balancing redox reactions in aqueous solutions
Rules for balancing redox reactions in acidic solutions
Rules for balancing redox reactions in basic solution
| The teacher guide learners to balance the following redox reactions.
The learners follow the teacher's guidelines SOLUTION
|
||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||
| EVALUATION 3 mins |
The teacher asks questions to the learners to evaluate the lesson.
|
Learners respond to the teacher's questions.
|
Ask the learners questions to assess the achievement of the set objectives.
|
||||||||||||||||||||||||||||||||||
| CONCLUSION 2mins |
The teacher guides the learners so that they come up with the concepts from the observations drawn. (Bridging the activities to the concept). | The two processes (oxidation and reduction) complement each other. If there is oxidation occurs, then there must also be a reduction, giving us the basis for this classification of a chemical reaction. Reactions involving an exchange of electrons are known as oxidation-reduction, simply, redox reactions. | Application: expansion of the concept | ||||||||||||||||||||||||||||||||||
| ASSIGNMENT |
|
Learners answer other questions. | Improving their level of understanding of Redox's reaction. | ||||||||||||||||||||||||||||||||||
 Oxidation of sulphur(IV) oxide
Oxidation of sulphur(IV) oxide
 Formation of margarine
Formation of margarine
 Catalysis Reaction
Catalysis Reaction
 Catalysis Reaction
Catalysis Reaction
 Catalysis Reaction
Catalysis Reaction
 Catalysis Reaction
Catalysis Reaction
 Reversible Reaction
Reversible Reaction