Lesson Plan and Lesson note on Physics
Models of the atom
Theme: Energy Quantization And Duality Of Matter
Topic: Models of the atom
Sub Topic:
Date: dd/mm/yyyy
Class: S.S 3
Average Age: 16 years and above
Duration: 40 Minutes
No of Learners: 40
At the end of the lesson, the students should be able to:
1. Explain the Concept of the Atom
(a) The concept of the atom is the fundamental idea that matter is composed of tiny, indivisible particles known as atoms.(b) The term "atom" comes from the Greek word "atomos," which means "indivisible." It was first proposed by ancient Greek philosophers like Democritus and Leucippus.
2. List and explain The models of the atom.
(i) Sir J. J. Thompson model
Thompson proposed an atomic model which visualized the atom as a homogenous sphere of positive charge inside of which are embedded negatively charged electrons.He also determined the ratio of the charged to mass, e/m, of electrons, and found e/m to be identical for all cathode rays particles, irrespective of the kind of gas in the tube or the metal the electrons are made of.
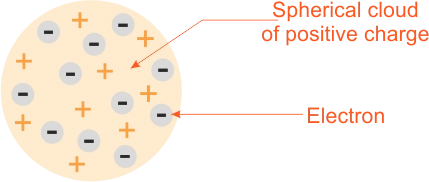 Sir J. J. Thompson atomic model
Sir J. J. Thompson atomic modelThompson’s model explains that:
(a) A normal atom is electrically neutral.
(b) Ions are formed by atom gaining or loosing electron.
(ii) Rutherford model
He proposed a planetary model of the atom which suggested that the atom consists of positively charged heave core called the nucleus where most of the mass of the atom was concentrated around this nucleus, negatively charged electrons circle in orbits much as planets move around the sun. Each nucleus must be surrounded by a number of electrons necessary to produce an electrically neutral atom.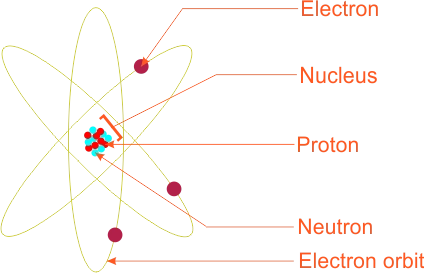 Rutherford atomic model
Rutherford atomic modelThe model was created to explain the back-scattering of alpha particles from thin metal foils. The postulates are statedas:
(a) The diameter of the atom is about 10−10 m.
(b) The diameter of the nucleus of an atom is 10−14 m.
(c) The region around the nucleus is large compare to the space occupied by th enucleus.
(d) The electrons are located around the nucleus.
(e) The mass of an atom is concentrated at the nucleus
(iii) Niels Bohr Model
Niels Bohr introduced the atomic Hydrogen model in 1913. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Niels Bohr atomic model
Niels Bohr atomic modelNiels Bohr suggested a model of hydrogen atom in which:
(a) The electrons in such stationary statements no radiation
(b) If an electron jumps to a lower state, it emits a photon whose energy equals the difference in energy between the two states.
(c) The angular momentum ‘L’ of the atomic electron is quantized by the rule (Where n=1, 2, 3, etc).
(d) The chemical and physical properties of an element depend on the number of electrons in the outermost shell.
(e) The chemical properties of element depend on the number of electrons in the outermost shell.
(iv) The electron cloud Model
This model visualizes the atom as consisting of a tiny nucleus of radius of the order of 10-10 m to 10-15 m. The electron is visualized as being in rapid motion within a relatively large region around the nucleus, but spending most of its time in certain high probability regions. Thus, the electron is not considered as a ball revolving around the nucleus but as a particle or wave with a specified energy having only a certain probability of being in a given region in the space outside the nucleus. The electron is visualized as spread out around the nucleus in a sort of electron–cloud.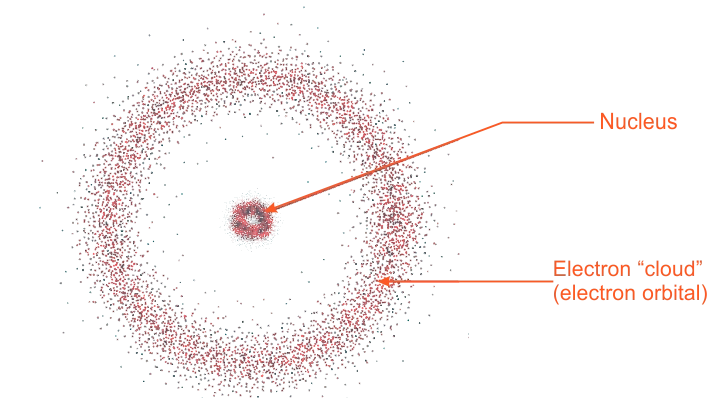 The electron cloud atomic model
The electron cloud atomic modelChemists prefer to consider the electron in terms of a cloud of negative charges (electron cloud), with a cloud being dense in regions of high electron probability and more diffuse in region of low probability.
The probability of finding the electron inside the spherical boundary is high. The probability then decreases rapidly as the distance of the thin shell from the nucleus increases.
3 List and explain the limitation of the atomic models.
(i) Limitation of Thompson’s model
(a) The scattering of alpha-particle by gold foil in Geiger and Marsden experiment.(b) The existence of line spectra of hydrogen atom and other complex gases.
(ii) Limitations of Rutherford model
(a) The assumed nature of the orbit not could be said to be fixed due to the electro static attraction of the electrons towards the nucleus by the positively charged protons.(b) The electrostatic force that is supposed to maintain the electrons in the irrespective orbits are weak due to emission of photon when accelerating electrons fall into the nucleus or from one orbit to the other.
(c) The model was unable to explain some observations behind the line of spectrum emitted by incandescent objects.
(iii) Limitations of Bohr’s model
(a) It does not explain how fixed orbits for electrons are chosen when they are not radiating energy.(b) The model is not applicable to the atom with more than one electron in the outermost shell.
(c) The model cannot be used to predict energy level for complex atoms with many electrons.
(iv) Limitation of electron cloud atomic model
(a) The model does not give the exact position of an electron in the orbital at a given time.(b) Electrons couldn't orbit the nucleus like miniature planets
(c) The electron cloud model, based on quantum mechanics, is the most accurate representation of the atom to date, but it can be conceptually challenging as it involves probability distributions rather than fixed orbits.
Rationale:
In order to understand the various chemical reactions that occur around us and the parameters governing these reactions, we need an understanding of the elements and compounds taking part in these reactions. The early developments in science revealed that matter is made up of atoms which in turn are composed of elementary particles known as electrons, protons and neutrons. In order to have a better understanding of the transformations of matter from one form to the other, scientists needed a defined structure for an atom and its interaction with other atoms.Prerequisite/ Previous knowledge:
Photoelectric Effect.Learning Resources:
Flash cards, an audio and video youtube examples, Available useful objects.Reference Materials:
1. New system physics for secondary school by Dr. Charles chew etal2. New school physics by M. W Anyakoha
3. Internet facility
Lesson Development:
| STAGE | TEACHER'S ACTIVITY | LEARNER'S ACTIVITY | LEARNING POINTS |
|---|---|---|---|
| STEP 1: INTRODUCTION Individual Student |
The teacher asks the students the following questions in Chemistry: 1. What is an Atom? 2. State Dalton’s Atomic Theory. 3. What are the Postulates of Dalton’s Atomic Theory? 4. What are the Limitations of Dalton’s Atomic Theory? 5. What are the Merits of Dalton’s Atomic Theory? |
The students expected answers:1. An atom is the basic building block of life. It is the smallest unit of matter that is composed of three sub-atomic particles: the proton, the neutron and the electron. It is the smallest unit into which matter can be divided without the release of electrically charged particles.2. Dalton’s Atomic TheoryDalton’s atomic theory stated that all matter was made up of small, indivisible particles known as ‘atoms’.3. Postulates of Dalton’s Atomic Theory(a) All matter is made up of tiny, indivisible particles called atoms.(b) All atoms of a specific element are identical in mass, size, and other properties. However, atoms of different element exhibit different properties and vary in mass and size. (c) Atoms can neither be created nor destroyed. Furthermore, atoms cannot be divided into smaller particles. (d) Atoms of different elements can combine with each other in fixed whole-number ratios in order to form compounds. (c) Atoms can be rearranged, combined, or separated in chemical reactions. 4. Limitations of Dalton’s Atomic Theory(a) It does not account for subatomic particles: Dalton’s atomic theory stated that atoms were indivisible. However, the discovery of subatomic particles (such as protons, electrons, and neutrons) disproved this postulate.(b) It does not account for isotopes: As per Dalton’s atomic theory, all atoms of an element have identical masses and densities. However, different isotopes of elements have different atomic masses (Example: hydrogen, deuterium, and tritium). (c) It does not account for isobars: This theory states that the masses of the atoms of two different elements must differ. However, it is possible for two different elements to share the same mass number. Such atoms are called isobars (Example: 40Ar and 40Ca). (d) Elements need not combine in simple, whole-number ratios to form compounds: Certain complex organic compounds do not feature simple ratios of constituent atoms. Example: sugar/sucrose (C11H22O11). (e) The theory does not account for allotropes: The differences in the properties of diamond and graphite, both of which contain only carbon, cannot be explained by Dalton’s atomic theory. 5. Merits of Dalton’s Atomic Theory(a) The law of multiple proportions, the law of conservation of mass, and the law of constant proportions are not violated by Dalton’s atomic theory.(b) The theory provides a basis to differentiate between elements and compounds. |
Identification of Prior Ideas and revising previous lesson |
| STEP 2: Development and Grouping |
The teacher asks students to form groups and choose their leaders and secretaries. Thereafter, the teacher’s leads the students to understand that Atomic orbitals are mathematical functions that provide insight into the wave nature of electrons (or pairs of electrons) that exist around the nuclei of atoms. In the fields of quantum mechanics and atomic theory, these mathematical functions are often employed in order to determine the probability of finding an electron (belonging to an atom) in a specific region around the nucleus of the atom. It is important to note that the term ‘atomic orbital’ can also be used to refer to the physical space or physical region around an atom’s nucleus in which the probability of a specific electron being present is maximum. The presence of an electron in such a region is predicted by the mathematical form of the atomic orbital. |
The students form groups and choose their leaders and secretaries. The students write down what the teacher explains and listen attentively. |
Inculcating leadership skills, competitive spirit, cooperation, teamwork and a sense of responsibility among learners. Develping the Concept of the topic Models of the atom |
|
| |||
| STEP 3: EXPLORATION Entire Class |
The teacher’s leads the students to understand Concept of the Atom. (a) The concept of the atom is the fundamental idea that matter is composed of tiny, indivisible particles known as atoms. (b) The term "atom" comes from the Greek word "atomos," which means "indivisible." It was first proposed by ancient Greek philosophers like Democritus and Leucippus. |
The students write down what the teacher explains and listen attentively. | Concept of the Atom. |
| STEP 3: DISCUSSION Entire class |
The teacher state/explain The models of the atom with the students.
(i) Sir J. J. Thompson modelThompson proposed an atomic model which visualized the atom as a homogenous sphere of positive charge inside of which are embedded negatively charged electrons.He also determined the ratio of the charged to mass, e/m, of electrons, and found e/m to be identical for all cathode rays particles, irrespective of the kind of gas in the tube or the metal the electrons are made of.  Sir J. J. Thompson atomic model
Sir J. J. Thompson atomic modelThompson’s model explains that: (a) A normal atom is electrically neutral. (b) Ions are formed by atom gaining or loosing electron. (ii) Rutherford modelHe proposed a planetary model of the atom which suggested that the atom consists of positively charged heave core called the nucleus where most of the mass of the atom was concentrated around this nucleus, negatively charged electrons circle in orbits much as planets move around the sun. Each nucleus must be surrounded by a number of electrons necessary to produce an electrically neutral atom. Rutherford atomic model
Rutherford atomic modelThe model was created to explain the back-scattering of alpha particles from thin metal foils. The postulates are statedas: (a) The diameter of the atom is about 10−10 m. (b) The diameter of the nucleus of an atom is 10−14 m. (c) The region around the nucleus is large compare to the space occupied by th enucleus. (d) The electrons are located around the nucleus. (e) The mass of an atom is concentrated at the nucleus (iii) Niels Bohr ModelNiels Bohr introduced the atomic Hydrogen model in 1913. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Niels Bohr atomic model
Niels Bohr atomic modelNiels Bohr suggested a model of hydrogen atom in which: (a) The electrons in such stationary statements no radiation (b) If an electron jumps to a lower state, it emits a photon whose energy equals the difference in energy between the two states. (c) The angular momentum ‘L’ of the atomic electron is quantized by the rule (Where n=1, 2, 3, etc). (d) The chemical and physical properties of an element depend on the number of electrons in the outermost shell. (e) The chemical properties of element depend on the number of electrons in the outermost shell. (iv) The electron cloud ModelThis model visualizes the atom as consisting of a tiny nucleus of radius of the order of 10-10 m to 10-15 m. The electron is visualized as being in rapid motion within a relatively large region around the nucleus, but spending most of its time in certain high probability regions. Thus, the electron is not considered as a ball revolving around the nucleus but as a particle or wave with a specified energy having only a certain probability of being in a given region in the space outside the nucleus. The electron is visualized as spread out around the nucleus in a sort of electron–cloud. The electron cloud atomic model
The electron cloud atomic modelChemists prefer to consider the electron in terms of a cloud of negative charges (electron cloud), with a cloud being dense in regions of high electron probability and more diffuse in region of low probability. The probability of finding the electron inside the spherical boundary is high. The probability then decreases rapidly as the distance of the thin shell from the nucleus increases. |
The students copied the worked examples. | Better understanding of the models of the atom |
The teacher explains the limitation of the atomic models with the students.(i) Limitation of Thompson’s model(a) The scattering of alpha-particle by gold foil in Geiger and Marsden experiment.(b) The existence of line spectra of hydrogen atom and other complex gases. (ii) Limitations of Rutherford model(a) The assumed nature of the orbit not could be said to be fixed due to the electro static attraction of the electrons towards the nucleus by the positively charged protons.(b) The electrostatic force that is supposed to maintain the electrons in the irrespective orbits are weak due to emission of photon when accelerating electrons fall into the nucleus or from one orbit to the other. (c) The model was unable to explain some observations behind the line of spectrum emitted by incandescent objects. (iii) Limitations of Bohr’s model(a) It does not explain how fixed orbits for electrons are chosen when they are not radiating energy.(b) The model is not applicable to the atom with more than one electron in the outermost shell. (c) The model cannot be used to predict energy level for complex atoms with many electrons. (iv) Limitation of electron cloud atomic model(a) The model does not give the exact position of an electron in the orbital at a given time.(b) Electrons couldn't orbit the nucleus like miniature planets (c) The electron cloud model, based on quantum mechanics, is the most accurate representation of the atom to date, but it can be conceptually challenging as it involves probability distributions rather than fixed orbits. |
The students write down the limitation of the atomic models and listen attentively to teacher. | Limitation of the atomic models | |
| STEP 4: APPLICATION Entire class |
The teacher ask the students to read through all they have copied and give more discussion/explanation as directed by the teacher. They take corrections where they are wrong. | The students did what the teacher ask them to do. | Better understanding of models of the atom. |
| STEP 5: EVALUATION Individual students |
The teacher asks the students questions to test them. 1. What is Niels Bohr’s Atomic Model? 2. What is the Limitations of Bohr Atomic Model Theory? 3. What is J. J. Thomsons Atomic Model And Its Limitations? |
The students respond to the questions correctly. SOLUTION 1. Niels Bohr proposed an atomic structure model, describing an atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the positively charged nucleus like planets around the sun in our solar system, with attraction provided by electrostatic forces, popularly known as Bohr’s atomic model. Salient features of Niels Bohr atomic model are: (i) Electrons revolve around the nucleus in stable orbits without emission of radiant energy. Each orbit has a definite energy and is called an energy shell or energy level. (ii) An orbit or energy level is designated as K, L, M, N shells. When the electron is in the lowest energy level, it is said to be in the ground state. (iii) An electron emits or absorbs energy when it jumps from one orbit or energy level to another. When it jumps from a higher energy level to lower energy level it emits energy while it absorbs energy when it jumps from a lower energy level to a higher energy level. (iv) The energy absorbed or emitted is equal to the difference between the energies of the two energy levels (E1, E2) and is determined by Plank’s equation. ΔE = E2-E1 = h𝜈 Where, ΔE = energy absorbed or emitted 2. Limitations of Bohr Atomic Model Theory (i) The Bohr atomic model theory considers electrons to have both a known radius and orbit i.e. known position and momentum at the same time, which is impossible according to Heisenberg. (ii) The Bohr atomic model theory made correct predictions for smaller sized atoms like hydrogen, but poor spectral predictions are obtained when larger atoms are considered. (iii) It failed to explain the Zeeman effect when the spectral line is split into several components in the presence of a magnetic field. (iv) It failed to explain the Stark effect when the spectral line gets split up into fine lines in the presence of an electric field. 3. Thomson proposed that the shape of an atom resembles that of a sphere having a radius of the order of 10-10 m. The positively charged particles are uniformly distributed with electrons arranged in such a manner that the atom is electrostatically stable. Thomson's atomic model was also called the plum pudding model or the watermelon model. The embedded electrons resembled the seed of a watermelon while the watermelon's red mass represented the positive charge distribution. The plum pudding atomic theory assumed that the mass of an atom is uniformly distributed all over the atom. Limitations of Thomson's Atomic Model |
Asking the learners questions to assess the achievement of the set objectives. |
| CONCLUSION | The teacher concluded the lesson Thomson’s atomic model and Rutherford’s atomic model failed to answer any questions related to the energy of an atom and its stability. |
The students write down the conclusion of the lesson and listen attentively. | Better understanding of Atomic Model. |
| ASSIGNMENT | The teacher gives learners take home. 1. What are the drawbacks of the rutherford atomic model? 2. What are the features of the rutherford atomic model? |
The learners copy the assignment | Better understanding of Models of the atom. |